Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation
Investigation of new modification strategies for PVA membranes
M.E. Dmitrenko a, A.V. Penkova a,⇑, A.I. Kuzminova a, M. Morshed b, M.I. Larionov a, H. Alem c, A.A. Zolotarev a,
S.S. Ermakov a, D. Roizard b
a St. Petersburg State University, 7/9 Universitetskaya nab., St. Petersburg 199034, Russia
b Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, ENSIC, 1 rue Granville, 54000 Nancy, France
c Université de Lorraine, CNRS, Institut Jean Lamour (IJL), UMR 7198, Nancy, France
articleinfo
Article history:
Received 10 January 2018
Revised 6 April 2018
Accepted 18 April 2018
Available online 22 April 2018
Keywords:
PVA
Pervaporation Fullerenol
Bulk modification Polyelectrolyte
Layer-by-layer deposition
abstract
Novel supported membranes based on polyvinyl alcohol (PVA) were developed using two strategies: first, by the modification of thePVA network, via so-called bulk modification, with the formation of the selec- tive layer accomplished through the introductionof fullerenol and/or poly(allylamine hydrochloride), and second, by the functionalization of the surface with successive deposit ons of multilayered films of polyelectrolytes, such as poly(allylamine hydrochloride) and poly(sodium 4-styrenesulfonate) on thePVA surface. The membrane surface modification was characterized by scanning electron microscopy and contact angle measurements. The modified PVA membranes were examined for their dehydration transport properties by the pervaporation of isopropyl alcoholwater (80/20% w/w), which was chosen as a model mixture. Compared with the pristine PVA membrane, the main improvement was a marked increase in permeability. It was found that the surface modification mainly gave rise to a higher permeation flux but with a strong reduction in selectivity. Only the combination of both bulk and surface modifications with PEL could significantly increase the flux with a high water content in the permeate (over 98%). Lastly, it should be noted that thisstudy developed a green procedure to prepare innovative membrane layers for dehydration, making use of only water as a working medium.
© 2018 Elsevier B.V. All rights reserved.
Introduction
PVA is a reference hydrophilic polymer known for its economic advantages, high selectivity to water and dehydration properties by pervaporation because of its good film-forming properties [1,2]. These reasons account for why PVA has already been used to prepare several series of commercial membranes [3]. Improving the properties of PVA membranes nevertheless remains a challenging task [4–7]. In this work, two distinct strategies to this end were investigated, i.e., bulk and surface modifications. Indeed, surface and bulk functionalizations can allow for the tailoring of the prop- erties of polymer materials [8–10]. These modification methods have already been applied in the field of membrane technology,
(Corresponding author.E-mail addresses: m.dmitrienko@spbu.ru (M.E. Dmitrenko), a.penkova@spbu.ru (A.V. Penkova), ai.kuzminova@mail.ru (A.I. Kuzminova), Mahbub.morshed@ univ-lorraine.fr (M. Morshed), Gra-viton@mail.ru (M.I. Larionov), halima.alem@ univ-lorraine.fr (H. Alem), andrey.zolotarev@spbu.ru (A.A. Zolotarev), s.ermakov@ spbu.ru (S.S. Ermakov), denis.roizard@univ-lorraine.fr (D. Roizard).https://doi.org/10.1016/j.apsusc.2018.04.1690169-4332/© 2018 Elsevier B.V. All rights reserved.)
since they help in developing membranes with improved parame- ters, such as anti-fouling properties and/or improved transport characteristics (flux, selectivity, permeance, barrier and mechani- cal properties) [11–14]. In particular, nonporous membranes are very sensitive to these modification procedures, which can affect the solution-diffusion mechanism behind gas separation and pervaporation.
The most suitable and prospective way to study and evaluate the effects of internal and surface modifications of a pervaporation membrane is to quantify the membrane mass-transfer. According to the solution-diffusion mechanism, there are three steps in mem- brane mass transfer:
(1) The upstream-side sorption, preferably favoring one of the components of the mixture in the membrane network. In this step, the membrane coating or surface functionalization can play a significant role.
(2) The diffusion of the components through the membrane. In this step, the available free volume linked to the bulk mem- brane modification can play a major role.
(3) The desorption of the components from the downstream side at low pressure. Usually, this step is assumed to be very fast; hence, it has a minor effect on the mass transfer.
Pervaporation is a well-known alternative method to classical distillation for the dehydration of alcohols when azeotropes are formed or when close-boiling component mixtures are considered. In such cases, pervaporation can provide substantially higher selectivity and thus a significant reduction in energy consumption [1,2]. The mixture of isopropyl alcohol (i-PrOH)-water is often studied as a model separation system for dehydration by pervapo- ration, since i-PrOH is an industrial solvent that can be used as a substitute for ethanol. i-PrOH is widely applied in such fields as perfumery, cosmetics, and medicine [15,16]. A 12 wt.% water – 88 wt.% isopropanol mixture forms an azeotropic mixture [17], which makes it difficult to dehydrate the alcohol by traditional separation methods (distillation and rectification). Using tradi- tional separation methods, it is necessary to add harmful organic solvents that form stronger azeotropic mixtures with water, which prohibits the production of a high-purity alcohol. In addition, these separation methods are energetically expensive. Therefore, a promising way of dehydrating isopropanol-water mixtures is per- vaporation, which can extract water through a membrane without any additional chemical reagents for dehydration. Various PVA membranes have already been widely used for the dehydration of isopropanol by pervaporation. However, to prevent strong swel- ling of the PVA in the aqueous solution and improve its stability, various methods for the modification or cross-linking of PVA have been attempted, for example, the creation of mixed-matrix blend membranes based on copolymers PVA/poly(N-isopropylacryla mide) (PNIPAAm) [18], PVA/sodium carboxymethylcellulose (NaCMC)/poly [19,20], and PVA/chitosan [21]; cross-linking with polyacrylic acid (PAA) [22], glutaraldehyde (GA) with concentrated HCl [19,20], oxalic acid (OA), dimethylol urea (DMU) and tetraethyl orthosilicate (TEOS) [23]; and the introduction of a zeolite or hydrophilic aluminosilicate filler into the PVA matrix [19,23]. However, PVA membrane performance still needs improvement for industrial separation processes.
A promising approach to improve the performance of membrane materials is the functionalization of membranes by the deposition of nanoscale layers on the surface of a selective polymer layer of the supported membrane [24,25]. The creation of an ultra- thin film on the surface can be realized by such classical methods as the Langmuir–Blodgett technique and the synthesis of self- organizing layers [26]. However, these approaches have significant drawbacks: first, the Langmuir–Blodgett technique requires expen- sive equipment to create layers, and this method is not applied to all polymers. Second, the self-assembled layer method is not suit- able or useful for multilayer fabrication [26]. To create a multi- layer film on the membrane surface, a relatively modern approach is applied, which is layer-by-layer (LbL) deposition [27–30]. This method is simple, inexpensive and suitable for many polymer materials; it is also easily automated. With this approach, various substances can be applied to the membrane material:polyelectrolytes[31], metallic nanoparticles [32], silicon nanoparticles[33] and many others. One of the promising directions to improvethe performance of membranes is the deposition of polyelec- trolytes onto the polymer film because of the unique properties of the deposited layers. Such a layered deposition leads to a charged film surface with a highly hydrophilic property, and conse- quently, a stronger affinity for water molecules. A dense electro- static layer should only cause a moderate swelling of these membranes while in contact with water, which makes polyelec- trolytes attractive for the functionalization and coating of pervapo- ration membranes [31].
The efficiency of pervaporation membranes can be significantly improved by varying the type of polyelectrolyte pairs and the application conditions (the deposited bilayer numbers, ionic strength and pH [34–36]). For example, alcohol/water pervapora- tion separation by polyelectrolyte multilayer membranes prepared via electrostatic layer-by-layer (LbL) adsorption of cationic (polyvinylamine (PVA)) and anionic (polyvinylsulfonate (PVS), polyvinylsulfate (PVS) and polyacrylate (PAA)) polyelectrolytes has been described [37]. It was shown that the hydrophilic PVA/ PVS membrane had optimal transport properties for the separation of a feed with low water content (<20 wt.%), while the less hydro- philic PVA/PAA membrane was suitable for the separation of mix- tures with higher water concentrations.
For better adhesion between the dense membrane and polyelectrolytes, an effective method that can be applied is the plasma treatment (e.g., O2 and Ar) of the pristine membrane surface to cre- ate negative charges. Films based on plasma-treated polydimethyl- siloxane (PDMS) have been further functionalized by the LbL deposition of more than 5 bilayers of poly (diallyldimethyl ammo- nium chloride) (PDADMAC) and poly(styrene sulfonate) (PSS) [38]. The optimal plasma treatment conditions for the films were chosen to obtain a full surface coating, resulting in defect-free and hydro- philic PDMS surfaces, as confirmed by SEM images and contact angle measurements.
This work aimed at improving the transport properties of PVA membranes for the dehydration of isopropanol by using two com- plementary strategies: bulk and surface modifications.
Several types of additives were considered for bulk modifica- tion, namely, fullerenol and poly(allylamine hydrochloride). Based on previous studies [39–43], fullerenol was chosen as one of the modifiers and cross-linking agents. During the chemical cross- linking of a membrane based on a PVA-fullerenol composite with maleic acid, the permeability of a membrane increased signifi- cantly with a slight decrease in selectivity during the separation of ethanol-water mixtures [42] because of the changes in the degree of crystallinity, surface polarity and free volume [42,41]. Poly(allylamine hydrochloride) has been used to improve the dis- persion of carbon nanoparticles as well as to increase the adhesion of nanolayers of polyelectrolytes deposited on a membrane surface by LbL assembly.
The surface modification of mixed-matrix PVA membranes was accomplished by LbL deposition coating with 10 or 20 bilayers of polyelectrolytes: poly(allylamine hydrochloride) as the polycation and poly(sodium 4-styrenesulfonate) as the polyanion. This modi- fication method is very promising for the functionalization of membrane surfaces with thin functionalized layers (10–100 nm) and can lead to significant changes of surface properties, such as an increased hydrophilicity, which can greatly modify the perfor- mance and transport characteristics of the membrane.
The transport properties of the membranes were studied with isopropyl alcohol (80 wt.%)-water (20 wt.%) feed mixtures in per- vaporation. Scanning electron microscopy and contact angle mea- surements were used to characterize the membrane surface before and after pervaporation experiments to evaluate the stability of the thin active layers. The transport properties of the developed mem- brane were compared with a commercially available analogous PVA membrane, i.e., PERVAPTM 1201, for isopropanol dehydration.
Materials and methods
Materials
The membrane material used was PVA with a molecular weight of 141 kDa from ZAO LenReaktiv (certificate of analysis № 5530413013, date of manufacture 09.2011). The polyhydroxylated fullerene, C60(OH)12 (Fullerene Technologies, Russia), was used for bulk PVA modification. Maleic acid (MA) from Sigma-Aldrich (France) with a purity of >99.0% was used as an additional cross- linking agent for the PVA membranes. Isopropyl alcohol (i-PrOH) obtained from Vekton (Russia) was used without additional treat- ment. Poly(allylamine hydrochloride) (PAH, Mw ~50,000) and poly (sodium 4-styrenesulfonate) (PSS, Mw 70,000) purchased from Sigma–Aldrich (France) were used as the cationic and anionic poly- electrolytes, respectively. For all of the experiments, deionized water (MilliQ® water) was used for the polyelectrolyte solutions.
A hydrophilic porous support based on an aromatic polysulfone amide (UPM, pore size 200 Å, from Vladipor, Russia) was chosen to prepare the supported membranes with a thin PVA top layer. The commercial supported membrane ‘‘PERVAPTM 1201” (a cross- linked PVA membrane for the dehydration of mixtures containing up to 80 wt.% water) was purchased from Sulzer Chemtec Co. and tested to compare transport parameters.
Preparation of supported membranes
PVA composites were prepared according to a previously reported procedure [42]: the required quantity of maleic acid (MA) (35% w/w with respect to the weight of the polymer), fullerenol (0 or 5% w/w with respect to the polymer weight) and/or poly- electrolyte (4.7% w/w with respect to the polymer weight) were added in a 2 wt.% PVA water solution using ultrasonic treatment with a frequency of 35 kHz for 40 min. The maximum loading of fullerenol was limited to 5 wt.%, with higher concentrations lead- ing to poor dispersion in membranes and causing defects and infe- rior mechanical properties.
All studied membranes were produced by casting a 2 wt.% aqueous solution of PVA with 35 wt.% MA or its composite solu- tions (PVA/polyelectrolyte/MA, PVA/fullerenol/MA, PVA/fullere nol/polyelectrolyte/MA) onto the surface of the commercial ultra- filtration support (UPM-20) and drying at room temperature for 24 h for solvent evaporation. The choice of the UPM-20 support was based on an earlier study [44] and was due to the good mechanical properties (maximum tension 26.52 ± 2.58 MPa, elas- tic modulus 0.44 ± 0.034 GPa, and maximum deformation 13.34± 3.51%) and chemical resistance of UPM-20 since other supports (including polyacrylonitrile (PAN)) could be hydrolyzed at high temperature. The thin-coated PVA layer had a thickness of 1 ±0.3 mm, as determined by scanning electron microscopy (SEM) measurements, and its cross-linking was achieved by heating the membrane at 110 °C for 120 min [42].
LbL deposition technique
PAH (10—2 mol/L) and PSS (10—2 mol/L) were used as the poly- electrolyte solutions. The pH of the PAH solution was adjusted to 4 because this polyelectrolyte is fully ionized at this value.
Multilayer PELs were deposited using a ND Multi Axis Dip Coater ND-3D 11/5 (Nadetech). This dip coater possessed a wide speed immersion range (from 1 to 2000 mm min—1) and ensured good reproducibility of the thin films.
The membrane was clamped and immersed in each PEL solution for 10 min. The polycation solution of PAH was deposited first and then the membrane was removed and rinsed thoroughly with water for 1 s/15 times, 5 s/3 times and 15 s/1 time, successively. After the membrane was immersed in the PSS solution for 10 min, the same water rinsing process of the membrane was repeated. The successive rinsing steps after the PEL deposition ensured the removal of excess polyelectrolyte and prevented cross-contamination of PEL solutions. In this way, one bilayer of polyelectrolyte on the surface of the membrane (or one cycle of selfassembly membrane) was completed. Similarly, additional bilayers were deposited until the required number of bilayers was reached. Because the polycation (PAH) was previously introduced into the polymer matrix, the polyanion (PSS) had been first depos- ited on the surface. The deposition consisted of 10 to 20 bilayers of polyelectrolytes (PSS/PAH) on the surface of the membrane.
Plasma treatment
Plasma surface modification was conducted using a microwave post-discharge reactor consisting of a cylindrical glass chamber (3 cm in diameter) pumped through a primary pump. The residual vacuum was 10—2 mbar. A 2.45 GHz microwave generator was used to generate the plasma. The plasma atmosphere consisted of an Ar and O2 gas mixture with a flow rate of 400 sccm and 40 sccm, respectively. Plasma gases were fed into the system via gas flow meters. The PVA sample was placed downstream from the plasma a 30 cm from the plasma outlet (Fig. 1). After the plasma treatment, the system was vented to atmospheric pressure, and then the sample was removed from the reactor. For the present study, the plasma power was 80 W, and the processing time was varied in a range from 30 s to 5 min. The optimum processing time was 2 min. As shown in Fig. 1, the plasma treatment was carried out using an Ar-O2 (10:1) plasma created in a 5 mm (id) quartz tube with a 2.45GHz microwave generator. This microwave power was optimized for effective surface membrane modification at 80 W [38]. All mod- ifications were conducted at 4 mbar. The post-discharge entered a 28 mm (id) Pyrex tube 30 cm downstream from the plasma gap.
IR spectroscopy
The spectra were recorded at 25 °C with a resolution of 1 cm—1 on an IR-Fourier spectrometer (BRUKER-TENSOR 27) from 400– 4000 cm—1.
Pervaporation experiment
A laboratory cell was used under steady-state conditions for the investigation of membrane transport properties at room tempera- ture (20 °C).
The pervaporation setup is presented in Fig. 2. The feed entered the cell (1). After separation by the membrane (2), the permeate (4) was condensed in a trap (3) cooled with liquid nitrogen (5). A downstream pressure of <10—1 kPa was achieved by a vacuum pump (7) and controlled by a pressure controller (6). The composi- tion of permeate and feed was analyzed by gas chromatography using a SHIMADZU GC-2010 chromatograph.
The membrane permeation flux, J (kg/(m2 h)), was calculated as the amount of liquid vaporized through a unit of the membrane area per hour and was calculated as (Eq. (1)) [45]:![]() J W 1A × t where W (kg) is the mass of the liquid that penetrated the mem- brane, A (m2) is the membrane area, and t (h) is the time of the measurement.
J W 1A × t where W (kg) is the mass of the liquid that penetrated the mem- brane, A (m2) is the membrane area, and t (h) is the time of the measurement.
Each measurement was carried out at least three times to ensure good accuracy of the transport parameters, i.e., ±0.5% for selectivity and ±2% for flux.
Scanning electron microscopy
SEM micrographs of the membrane cross-sections were obtained using a Zeiss Merlin scanning electron microscope. The supported membranes were submerged in liquid nitrogen and fractured perpendicular to the surface. The prepared samples were observed using SEM at 1 kV.
(Fig. 1. Schematic representation of the plasma afterglow reactor.) (Fig. 2. Scheme of the pervaporation set-up: 1-cell with the feed, 2-membrane, 3-cold trap, 4-permeate, 5-trap with liquid nitrogen, 6-pressure controller, and 7-vacuum pump.)
(Fig. 2. Scheme of the pervaporation set-up: 1-cell with the feed, 2-membrane, 3-cold trap, 4-permeate, 5-trap with liquid nitrogen, 6-pressure controller, and 7-vacuum pump.)
Contact angle determination
The contact angle measurements of the thin selective layer of the supported membranes were carried out as described in [46] to study the change in the surface properties (hydrophilicity) dur- ing modification.
Total organic carbon (TOC) analysis
The TOC measurements were carried out by a TOC analyzer (Shimadzu TOC-VCSH). The sensitivity of the apparatus was in the 10—2 ppm range. The membranes were placed into deionized water to carry out a drip washing test (up to 24 h). Samples of the water were analyzed to measure the carbon concentration (mg/L). The concentration of carbon in water was measured by the combustion of the sample in the combustion gas, followed by carbon dioxide analysis in the IR chamber. Several injections of the sample were made to obtain an average value and to calculate the standard deviation (SD) and the variation coefficient (CV). If the SD was higher than 0.1 or the CV was higher than 2%, then the measurement was repeated.
Results and discussion
Transport properties after membrane modification
The application of bulk and/or surface modifications was expected to significantly improve the membrane separation prop- erties. One of the ways to change the polymer free volume (bulk), and at the same time, to functionalize the selective top surface is through the creation of a mixed–matrix membrane. In previous studies, we found that the addition of fullerenol to PVA has led to an increase in surface hydrophilicity (shown by contact angle measurements), a decrease in the free volume (shown by pervapo- ration results) and a change in crystallinity (shown by X-ray) [39,42,41,44].
For this study, the chosen reference membranes were chemi- cally cross-linked supported membranes based on PVA and a PVA-fullerenol (5%) composite. The supported membrane contain- ing fullerenol was found to exhibit the best transport properties for the dehydration process, as shown in previous investigations [39,42,41,44]. For these chemically cross-linked supported mem- branes, two new modification approaches were developed: surface modification, using plasma treatment and layer-by-layer (LbL) deposition of polyelectrolytes, and bulk modification, which intro- duces nanoparticles of PAH into the polymer matrix.
Plasma modification
One of the effective and promising methods to increase the adhesion of a polyelectrolyte nanolayer to a membrane surface is plasma modification. Thisprocess can be considered as a method of surface pretreatment; in industry, it is widely used for treating surfaces of various materials before printing,gluing, coating or adhesion. Plasma treatment may also remove any contaminants from the surface, and in some cases, change the chemical structureof the surface. Thus, this surface modification by plasma treatment can significantly affect the characteristics of the thin selective PVA layer of the membranes. For this paper, the membrane plasma treatment was performed according to a procedure published pre- viously using plasma obtained from oxygen (O2) and argon (Ar) gases. The modified membranes were then investigated for the separation of i-PrOH/water mixture (80–20 wt.%) by pervaporation at 20 °C to evaluate the influence of the plasma treatment. The structure of the selective thin PVA layer of the supported mem- branes treated byplasma was investigated before and after perva- poration by IR spectroscopy to study the stability of membrane surface during the dehydration processof i-PrOH. Additionally, the spectrum of the untreated PVA membrane was obtained for comparison with a plasma-treated membrane to study the change in the structure of PVA under plasma treatment.
In Fig. 3, the IR spectra of the PVA membrane (Fig. 3(a)), and a PVA membrane after plasma treatment for 2 min before and after the pervaporation experiment (Fig. 3(b, c)) are presented to study the changes in the structure of the pristine membrane after plasma treatment.
It was found that the spectra in (b) and (c) are nearly identical, which indicates the stability of the PVA membrane structure trea- ted by plasma during pervaporation.
The following features were observed in the IR spectra of the PVA membranes (Fig. 3):
– The wide bands at 3300 and 1660 cm—1 refer to the vibrations of trace H2O.
– The stretching vibrations of the CH2 bonds correspond to the absorption in the 2800–3000 cm—1 region, the deformation vibrations of the ACH2 groups appear in the region of 718 cm—1 and are located in the spectral region 1150–1350 cm—1 [47]. The absorption bands with maxima at 1100 and 1300 cm—1, according to [48], correspond to vibrations associated with the CAOAH group.
Substantial changes in the IR spectra were observed after the plasma treatment of PVA: (1) a consecutive decrease is observed in the intensity of the bands (in the row: PVA membrane (Fig. 3 (a)), PVA membrane treated by plasma before pervaporation (Fig. 3(b)) and after pervaporation (Fig.3(c))) and (2) the position of individual bands (for untreated and plasma-treated membranes) that are attributed to stretching vibrations of AOH and ACH bonds, leading to their almost complete disappearance due to the dehy- dration and oxidation of the PVA molecule under O2/Ar plasma treatment.
The transport properties of the untreated and plasma-treated membranes are presented in the Table 1.
As can be seen from Table 1, the plasma treatment applied to the PVA membrane leads to a marked reduction of the pervapora- tion flux without any improvement in the selectivity compared with the pristine membrane, which could be caused by the oxida- tion and dehydration of PVA.
Earlier studies have shown that the introduction of fullerenol to the PVA matrix led to an increase in the membrane selectivity property with respect to water because of changes in membrane structure and crystallinity [39,42,41]. Therefore, the modification of the PVA membrane by fullerenol was carried out in the present work, and the modified membrane was subjected to the plasma treatment. The transport properties of the obtained membranes for the separation of the i-PrOH/water mixture (20 wt.% water,80 wt.% i-PrOH) at 20 °C are also presented in the Table 1. Similar to the membranes based on pure PVA, a decrease in flux was observed: the composite PVA-fullerenol (5%) membrane after plasma treatment exhibited a flux reduced by a factor 3 with comparable water selectivity. No significant drop in selectivity was observed compared with those of the PVA membranes without fullerenol, which was due to the change in the surface property of the membranes (increased hydrophilicity of the surface because of the increased number OH-groups), as well as a greater cross- linking of PVA chains because of the fullerenol modification [42].
These results are in good agreement with a previous study on other polymers showing that oxygen plasma treatment can lead to the etching of the polymer surface [49], which could lead to a decrease in the flux and selectivity towards water molecules of PVA membranes. Thus, it is confirmed that the plasma treatment of PVA membranes is not an effective modification method to pre- pare PEL-modified membranes.
LbL deposition
Taking into account the above results, the layer-by-layer (LbL) technique of polyelectrolyte deposition was applied directly with- out any plasma treatment to further enhance the charge density of the PVA surface and favor the transport of water molecules. The surface of the mixed-matrix PVA membranes presented in this sec- tion were coated with 10 or 20 bilayers of polyelectrolytes: poly (allylamine hydrochloride) as the polycation (noted PAH) and poly(sodium 4-styrenesulfonate) as the polyanion (noted PSS). The pervaporation transport properties of these membranes pre- sented in Fig. 4 were obtained with the model mixture i-PrOH(80 wt.%) – water (20 wt.%) at 20 °C.
The results of Fig. 4 (a) show that the surface modification by deposition of 10 PEL bilayers leads to an increase in flux for the two types of membranes compared with those of the pristine PVA and PVA-fullerenol (5%) membranes. However, the water selectivity is significantly reduced for the supported membranes after the LbL deposition (the water contents in the permeate are 65.7 and 85.6 wt.%) (Fig. 4 (b)). The PVA-fullerenol (5%)/LbL-10 membrane has a high water selectivity compared with that of the PVA/LbL-10 membrane because of the introduction of fullere- nol into the polymer matrix, as fullerenol is both a modifier and a cross-linking agent. The increase in the flux for the PVA and PVA-fullerenol (5%) membranes containing 10 PEL layers can be ascribed to the formation of small hydrophilic mashes induced by the higher charge density of PEL that can favor the penetration of water molecules versus the larger, less polar i-PrOH molecules [34]. To further modify the transport properties of the composite Fig. 3. The IR spectra of the pristine PVA membrane (a), PVA/plasma treatment membrane before pervaporation (b) and the membrane after pervaporation (PV) (c).
Fig. 3. The IR spectra of the pristine PVA membrane (a), PVA/plasma treatment membrane before pervaporation (b) and the membrane after pervaporation (PV) (c).

membranes on the UPM support and to investigate the dependence of the i-PrOH/water separation transport characteristics on the bilayer number, the number of polyelectrolyte bilayers was increased up to 20. The data presented in Fig. 4 show that, con-versely to the first case, the increase in the number of polyelec- trolyte bilayers up to 20 led to a decrease in the PVA-fullerenol (5%)/LbL-20 membrane flux compared with those of the pristine membranes (Fig. 4(A)). Meanwhile, the separation factor for water was not improved over the case of the 10-bilayer sample (a water concentration in the permeate of approximately71.4 wt.%). Thus, the 10 PEL bilayer coating on the membrane surface obtained by LbL deposition was chosen as the preferred surface modification for testing the strategy of bulk modification by PELs to improve the membrane dehydration properties. The decrease in the trans- port properties of the membranes with 20 PEL bilayers could be explained by the following factors. It is known that the membrane flux is inversely proportional to the PEL charge density for polar solutes [34]. The depositionof 20 PEL bilayers on the PVA mem- brane surface led to an increase in the thickness of the PEL layer exceeding 60 nm. This led to an increase in the PEL cross-linkingdensity and to an inhibited diffusion path for the penetrants in the PEL layerto the selective layer based on PVA, ultimately lead- ing to a decrease in the membrane flux. On the other hand, the for- mation of the polar mashes in the PEL layer facilitated water sorption by the membrane as well as an increase in surface hydrophilicity, leading to increased water content in the PEL layers that simultaneously caused i-PrOH penetration and a decrease in selectivity.
Bulk modification of the PVA network with a polyelectrolyte
The mass transfer through a membrane is closely related to the available free volume in the active layer. Therefore, a way to increase this free volume without overly reducing selectivity is to introduce modifications of the polymer network at the nanoscale, for instance, by introducing nanoparticles or by adding another type of polymer chain to the pristine polymer matrix. The expected effect was a decrease in the strength of the hydrogen bonding of the PVA network while keeping the polar character of the matrix. Therefore, poly(allylamine hydrochloride) (PAH) was chosen for further investigation. In addition, the introduction of PAH should contribute to the stabilization of the PEL bilayer through interac- tions with the PSS component of the PEL bilayers.
Mixed-matrix membranes based on PVA modified by fullerenol (5 wt.%) and/or PAH (4.7 wt.%) were prepared for this section. The
Fig. 4. Dependence of flux (A) and water content in the permeate (B) on the modification method (pristine, LbL deposition of 10 bilayers (LbL-10) or 20 bilayers (LbL-20)) of the supported membrane based on PVA or composite PVA-fullerenol (5%) during the pervaporation of a i-PrOH/water mixture (20 wt.% water, 80 wt.% i-PrOH) at 20 °C.
transport characteristics of the obtained supported membranes based on PVA and its composites were determined by pervapora- tion of the binary mixture isopropanol (80 wt.%) – water (20 wt.%) at 20 °C. The results are presented in Table 2.
The data presented in the table show that the introduction of PAH into the bulk PVA polymer matrix led to a marked increase in flux ( 2 times) compared with that of the pristine PVA mem- brane; however, at the same time, a significant decrease in the water content in the permeate was observed. This fact is attributed to polar PAH chains that disrupted the H-bonding organization of PVA. Compared with the introduction of fullerenol into PVA (Table 1, Section 3.1.1), the PAH effect on mass transfer appeared to be stronger. The combined dispersion of PAH and fullerenol into the PVA matrix also resulted in an increase in flux compared with the unmodified membrane, as well as an increase in water selectiv- ity (99.1 wt.% in permeate), because of the cross-linking of the PVA matrix by the fullerenol. A surface modification by the LbL deposi- tion was applied to the supported membranes to further improve the transport properties. It was shown that the membranes based on the PVA-PAH and PVA-fullerenol-PAH composites after the LbL modification have higher flux values than those of the PVA/LbL-10 and PVA-fullerenol (5%)/LbL-10 membranes (Fig. 4) with signifi- cantly improved water selectivity values, especially for the com- posite membranes with fullerenol and PAH. Only for the dispersion with two modifiers (fullerenol and PAH) in the polymer matrix and after the LbL deposition of 10 bilayers a high water con- tent in the permeate (98.4 wt.%) and a high flux (0.286 kg/(m2 h)) could be obtained.
Thus, on the basis of the performed experiments, it was established that the mixed-matrix membrane based on the composite PVA-fullerenol (5%)-PAH (4.7%)-MA (35%) supported on UPM and modified with 10 polyelectrolyte bilayers by LbL deposition (membrane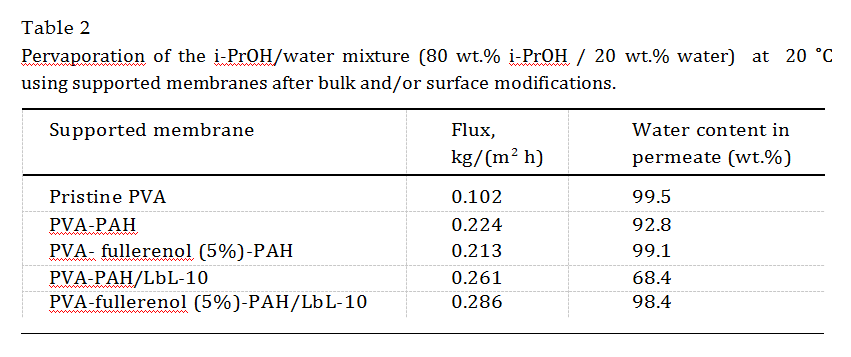
PVA-fullerenol (5%)-PAH/LbL-10) has the best transport properties for the dehydration of i-PrOH (20 wt.% water, 80 wt.% i-PrOH) by pervaporation.
Study of the stability of the supported membrane with the selective PVA-fullerenol (5%)-PAH/LbL-10 layer
To verify the stability of the polyelectrolyte nanosized multi- layer in the PVA-fullerenol(5%)-PAH/LbL-10 membrane on the UPM support, contact angles were measured by the static sessile drop method, and the membrane morphology was studied by scan- ning electron microscopy (SEM).
The optical and SEM images at different magnifications of the PVA-fullerenol (5%)-PAH/LbL-10 membrane are presented in Fig. 5. The presented images (Fig. 5) demonstrate the absence of defects andcontinuity of the surface and cross-section of the thin selective layer and PEL layers. It can be seen that PVA-fullerenol (5%)-PAH/LbL-10 membrane has a flat homogeneous membrane surface without any defects or distortions. The pictures of the membrane cross-section demonstrate the continuity and unifor- mity of the distribution of the thin selective PVA layer and the upper PEL layer.
Additional cross-sectional SEM micrographs were taken for the PVA-fullerenol (5%)-PAH membrane (Fig. 6(a)), as well as the PVA- fullerenol (5%)-PAH/LbL-10 membrane before (Fig. 6(b)) and after pervaporation (Fig. 6(c)), to demonstrate theexistence and the sta-bility of the PEL bilayers.
In the cross-sectional SEM micrograph of the supported mem- brane PVA-fullerenol(5%)-PAH membrane (Fig. 6 (a)), only two dis- tinguished areas were noticed: (1) the region of the porous UPM support and (2) the selective thin non-porous PVA layer; while the PVA-fullerenol(5%)-PAH/LbL-10 membrane modified by 10 PEL bilayers (Fig. 6 (b, c))contained an additional area (3) that cor- responds to the polyelectrolyte layer. A similar white nanosized PEL layer has also been demonstrated by Klitzing et al. [35] (cf. Fig. 12, p. 194). The size of the polyelectrolyte multilayer was determined to be 60 nm by SEM. The presented images for the PVA-fullerenol(5%)-PAH/LbL-10 membrane before (b) and after (c) pervaporation are identical, which shows that there were no changes to the selective PVA layer and the deposited polyelec- trolyte layers before and after pervaporation. As expected, the layer-by-layer assembly is stable upon contact with water-organic solutions. Fig. 5. Optical and SEM images of the PVA-fullerenol (5%)-PAH/LbL-10 membrane.
Fig. 5. Optical and SEM images of the PVA-fullerenol (5%)-PAH/LbL-10 membrane.
Another confirmation of the stability of the thin PEL layer was obtained from the unchanged surface properties of the membrane: the contact angle measurements demonstrated the same values for water before and after the pervaporation experiments, equal to (72 ± 3)° and (72 ± 1)°, respectively. These data show that the contact angle of the membrane is not altered by the pervaporation experiment, which proves that the polyelectrolyte layers are anchored onto the surface of the PVA membrane.
Finally, the water stability of the PEL layer created on the PVA was tested in pure water to understand if the water concentration in thefeed could be an issue or not. For this purpose, tentative extractions/ dissolutions of the PEL layer by immersion in pure water (neutral pH, 24 h in deionized water) were carried out, followed by TOC analysis of the used water. It must be emphasized that these experiments, while simple in theory, are, from a practical point of view, difficult to carry out because of the tedious control of any carbon pollution linked to the experiments and to the pristine PVA-supported membrane as well. However, regardless of the water sample, after immersion, no significant carbon peak could be detected after 10 days. The successive measurements gave an extremely low value of carbon, comparable with those of the blank water samples. The TOC analysis demonstrated that thecarbon content in water does not exceed the measurement error (0.013 mg/L), which indicated the stability of the membrane in water. The SEM micrograph of the membrane cross-section, obtained after the immersion experiment and the TOC measurement, is shown in Fig. 7.
The SEM image shows the presence of three layers, i.e., the UPM, the PVA-fullerenol(5%)-PAH and the LbL-10 layer, after aging in water, as described above (Fig. 6).
Additionally, these results allow one to make the conclusion that the stability of the PAH/PSS layers strongly depends on the kind of substrate used for their deposition and on the adjusted pH. Indeed, it has been reported that this pair of polyelectrolytes deposited on a porous substrate based on polyethylene terephtha- late fleece with polyacrylonitrile decomposed when the water con- tent in the feed mixture was higher than 20 wt.% [34] at pH = 2.1. However, the high stability of the PAH/PSS layers was sustained when a nonporous polyvinyl alcohol-based system was used as a support layer at pH = 4.
Comparison of the transport properties of the supported membrane with the selective PVA-fullerenol (5%)-PAH/LbL-10 layer with those of the commercially available analog, PervapTM 1201
The transport properties of the best supported PVA-fullerenol (5%)-PAH/LbL-10 membrane were compared with the comme Fig. 6. Cross-sectional SEM micrographs of the supported membranes: PVA-fullerenol(5%)-PAH (a), and PVA-fullerenol(5%)-PAH/LbL-10 before (b) and after (c) pervaporation.
Fig. 6. Cross-sectional SEM micrographs of the supported membranes: PVA-fullerenol(5%)-PAH (a), and PVA-fullerenol(5%)-PAH/LbL-10 before (b) and after (c) pervaporation.
 Fig. 7. SEM micrograph of the cross-section of the supported PVA-fullerenol(5%)- PAH/LbL-10 membrane after immersion in water (TOC experiment)
Fig. 7. SEM micrograph of the cross-section of the supported PVA-fullerenol(5%)- PAH/LbL-10 membrane after immersion in water (TOC experiment)
cially available analog – the supported membrane ‘‘PERVAPTM 1201” from the company Sulzer Chemtech. The manufacturer reports that this type of membrane allows the dehydration of organic mixtures with a water content up to 80 wt.%. The commercial membrane PERVAPTM 1201 and PERVAPTM 1201/LbL-10 (mod- ified by PEL deposition) were tested for pervaporation of the same i-PrOH/water mixture (80/20 w/w) at 20 °C. A comparison of the transport properties of the obtained membranes during the separa- tion of the isopropanol-water mixture is presented in Table 3.
The PERVAPTM 1201 exhibits a very high selectivity but also a very low flux. The application of the PEL coating on this commer- cially available membrane did not lead to significant changes in the transport properties of the membrane compared with those of the original membrane (a slight difference in the fluxes with the same water selectivity). This should be linked to the high cross-linking density of the pristine polymer network. These data reveal that the flux of the developed PVA-fullerenol (5%)-PAH/ LbL-10 supported membrane exceeds the flux of the PERVAPTM 1201 membrane by 8.5 times and the flux of pristine composite PVA- fullerenol (5%) membrane by 2.4 times while allowing a high selectivity level (a water content in the permeate of 98.4 wt.%).
Accordingly, it can be concluded that the developed PVA-fullerenol (5%)-PAH/LbL-10 supported membrane possesses improved transport characteristics compared with those of PVA and PERVAPTM 1201. The modification strategies are therefore quite promising and give an interesting perspective for developing new green pervaporation membranes.
Conclusions
In this study, it was shown that the simultaneous application of bulk and surface modifications is a promising strategy to create high-performance mixed-matrix membranes for dehydration by pervaporation.
The application of a plasma surface pretreatment to PVA, for improving the adhesion of PEL layers to the membrane surface, should be avoided, because it leads to a significant decrease in the membrane flux. However, even without plasma pretreatment, a thin PEL coating stable to pervaporation conditions could be obtained. This was shown by consistent properties, SEM views and comparable contact angle measurements after PV experiments.
It was found that the preferred number of LbL deposited PEL (PAH/PSS) layers on the surface of composite PVA-supported mem- branes was 10 bilayers since an increase to 20 PEL bilayers led to a significant decrease in selectivity and flux. The decrease in trans- port properties was explained by an increase in the surface polarity leading to increased water content in the PEL layers.
It was shown that the best transport characteristics for perva- poration dehydration were achieved through the combination of two modification methods: the use of bulk modification (the intro- duction of two modifiers, fullerenol and PAH, into the PVA matrix) and surface modification (LbL deposition of 10 PEL bilayers) tech- niques. The highly effective mixed-matrix supported membrane based on composite PVA-fullerenol (5%)-PAH (4.7%)-MA (35%) with a modified surface coating of 10 PEL bilayers was developed. The obtained membrane had a flux 8.5 times higher than that of the commercially available analog PERVAPTM 1201 (Sulzer) for the per- vaporation separation of the i-PrOH-water mixture.
Acknowledgment
This work was supported by the Russian Science Foundation [Grant No. 17-73-20060]. The experimental work of this study was facilitated by CNRS resources and equipment from the Resource Centers of GEOMODEL, Center for X-ray Diffraction Meth- ods, Centre for Innovative Technologies of Composite Nanomateri- als, the Chemical Analysis and Materials Research Centre, and the Interdisciplinary Resource Center for Nanotechnology at St. Peters- burg State University.
References:
[1] B. Bolto, M. Hoang, Z. Xie, A review of membrane selection for the dehydration of aqueous ethanol by pervaporation, Chem. Eng. Process. Process Intensif. 50 (2011) 227–235, https://doi.org/10.1016/j.cep.2011.01.003.
[2] X. Zhao, L. Lv, W. Zhang, S. Zhang, Q. Zhang, Polymer-supported nanocomposites for environmental application: a review, Chem. Eng. J. 170 (2011) 381–394, https://doi.org/10.1016/j.cej.2011.02.071.
[3] S. Chemtech, Membrane Technol. (2006), https://doi.org/10.1002/ 3527608788.
[4] W. Yave, The improved pervaporation PERVAP membranes, Filtr. Sep. 54 (2017) 14–15, https://doi.org/10.1016/S0015-1882(17)30126-X.
[5] A. Khazaei, V. Mohebbi, R.M. Behbahani, A. Ramazani, S.A., Pervaporation of toluene and iso-octane through poly(vinyl alcohol)/graphene oxide nanoplate mixed matrix membranes: Comparison of crosslinked and noncrosslinked membranes, J. Appl. Polym. Sci. 135 (2018) 45853, https://doi.org/10.1002/ app.45853.
[6] S.G. Chaudhri, J.C. Chaudhari, P.S. Singh, Fabrication of efficient pervaporation desalination membrane by reinforcement of poly(vinyl alcohol)-silica film on porous polysulfone hollow fiber, J. Appl. Polym. Sci. 135 (2018) 45718, https:// doi.org/10.1002/app.45718.
[7] A. Malekpour, B. Mostajeran, G.A. Koohmareh, Pervaporation dehydration of binary and ternary mixtures of acetone, isopropanol and water using polyvinyl alcohol/zeolite membranes, Chem. Eng. Process. Process Intensif. 118 (2017) 47–53, https://doi.org/10.1016/j.cep.2017.04.019.
[8] Y. Zhao, K.W.K. Yeung, P.K. Chu, Functionalization of biomedical materials using plasma and related technologies, Appl. Surf. Sci. 310 (2014) 11–18, https://doi.org/10.1016/j.apsusc.2014.02.168.
[9] F. Asvadi, A. Raisi, A. Aroujalian, Preparation of multi-layer pervaporation membrane by electro-spraying of nano zeolite X, Microporous Mesoporous Mater. 251 (2017) 135–145, https://doi.org/10.1016/j.micromeso.2017.05.060.
[10] D. Hetemi, J. Pinson, Surface functionalisation of polymers, Chem. Soc. Rev. 46 (2017) 5701–5713, https://doi.org/10.1039/C7CS00150A.
[11] Q. Nan, P. Li, B. Cao, Applied Surface Science Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination, Appl. Surf. Sci. 387 (2016) 521–528, https://doi.org/10.1016/j.apsusc.2016.06.150.
[12] W. Zhang, Z. Yu, Q. Qian, Z. Zhang, X. Wang, Improving the pervaporation performance of the glutaraldehyde crosslinked chitosan membrane by simultaneously changing its surface and bulk structure, J. Membr. Sci. 348 (2010) 213–223, https://doi.org/10.1016/j.memsci.2009.11.003.
[13] Q.W. Yeang, S.H.S. Zein, A.B. Sulong, S.H. Tan, Comparison of the pervaporation performance of various types of carbon nanotube-based nanocomposites in the dehydration of acetone, Sep. Purif. Technol. 107 (2013) 252–263, https:// doi.org/10.1016/j.seppur.2013.01.031.
[14] D. Hua, T.-S. Chung, Polyelectrolyte functionalized lamellar graphene oxide membranes on polypropylene support for organic solvent nanofiltration, Carbon N. Y. 122 (2017) 604–613, https://doi.org/10.1016/ j.carbon.2017.07.011.
[15] E. Fu, K. McCue, D. Boesenberg, Chemical Disinfection of Hard Surfaces – Household, Industrial and Institutional Settings, Handb. Cleaning/ Decontamination Surfaces, Elsevier, 2007, pp. 573–592, https://doi.org/ 10.1016/B978-044451664-0/50017-6.
[16] R.P. Mlcak, S.D. Hegde, D.N. Herndon, Respiratory Care, Total Burn Care e2, Elsevier, 2012, pp. 239–248, https://doi.org/10.1016/B978-1-4377-2786- 9.00020-5.
[17] S.K. Ogorodnikov, T.M. Lesteva, V.B. Kogan, Azeotrope mixtures, Chemistry, St. Petersburg, 1971.
[18] F. Kurs,un, N. Is,ıklan, Development of thermo-responsive poly(vinyl alcohol)-g- poly(N-isopropylacrylamide) copolymeric membranes for separation of isopropyl alcohol/water mixtures via pervaporation, J. Ind. Eng. Chem. 41 (2016) 91–104, https://doi.org/10.1016/j.jiec.2016.07.011.
[19] C.V. Prasad, B. Yeriswamy, H. Sudhakar, P. Sudhakara, M.C.S. Subha, J.I. Song, et al., Preparation and characterization of nanoparticle-filled, mixed-matrix membranes for the pervaporation dehydration of isopropyl alcohol, J. Appl. Polym. Sci. 125 (2012) 3351–3360, https://doi.org/10.1002/app.35658.
[20] C.V. Prasad, H. Sudhakar, B. Yerri Swamy, G.V. Reddy, C.L.N. Reddy, C. Suryanarayana, et al., Miscibility studies of sodium carboxymethylcellulose/ poly(vinyl alcohol) blend membranes for pervaporation dehydration of isopropyl alcohol, J. Appl. Polym. Sci. 120 (2011) 2271–2281, https://doi.org/ 10.1002/app.33418.
[21] N. Ghobadi, T. Mohammadi, N. Kasiri, M. Kazemimoghadam, Modified poly (vinyl alcohol)/chitosan blended membranes for isopropanol dehydration via pervaporation: synthesis optimization and modeling by response surface methodology, J. Appl. Polym. Sci. 134 (2017), https://doi.org/10.1002/ app.44587.
[22] N.D. Hilmioglu, S. Tulbentci, Pervaporative separation of isopropyl alcohol/ water mixtures: effects of the operation conditions, Desalin. Water Treat. 48 (2012) 191–198, https://doi.org/10.1080/19443994.2012.698812.
[23] P. Das, S.K. Ray, S.B. Kuila, H.S. Samanta, N.R. Singha, Systematic choice of crosslinker and filler for pervaporation membrane: a case study with dehydration of isopropyl alcohol–water mixtures by polyvinyl alcohol membranes, Sep. Purif. Technol. 81 (2011) 159–173, https://doi.org/10.1016/ j.seppur.2011.07.020.
[24] J.-Y. Lee, Q. She, F. Huo, C.Y. Tang, Metal–organic framework-based porous matrix membranes for improving mass transfer in forward osmosis membranes, J. Membr. Sci. 492 (2015) 392–399, https://doi.org/10.1016/j. memsci.2015.06.003.
[25] J. Zhao, Y. Zhu, F. Pan, G. He, C. Fang, K. Cao, et al., Fabricating graphene oxide- based ultrathin hybrid membrane for pervaporation dehydration via layer-by- layer self-assembly driven by multiple interactions, J. Membr. Sci. 487 (2015) 162–172, https://doi.org/10.1016/j.memsci.2015.03.073.
[26] K. Ariga, J.P. Hill, Q. Ji, Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application, PCCP 9 (2007), https://doi.org/10.1039/b700410a.
[27] J. Yu, S. Cheng, Q. Che, Preparation and characterization of layer-by-layer self- assembly membrane based on sulfonated polyetheretherketone and polyurethane for high-temperature proton exchange membrane, J. Polym. Sci., Part A: Polym. Chem. 55 (2017) 3446–3454, https://doi.org/10.1002/ pola.28725.
[28] C.J. Lefaux, B.-S. Kim, N. Venkat, P.T. Mather, Molecular composite coatings on nafion using layer-by-layer self-assembly, ACS Appl. Mater. Interfaces. 7 (2015) 10365–10373, https://doi.org/10.1021/acsami.5b01371.
[29] S. Zuin, P. Scanferla, A. Brunelli, A. Marcomini, J.E. Wong, W. Wennekes, et al., Layer-by-layer deposition of titanium dioxide nanoparticles on polymeric membranes: a life cycle assessment study, Ind. Eng. Chem. Res. 52 (2013) 13979–13990, https://doi.org/10.1021/ie302979d.
[30] E.S. Dragan, M. Mihai, J. Schauer, L. Ghimici, PAN composite membrane with different solvent affinities controlled by surface modification methods, J. Polym. Sci., Part A: Polym. Chem. 43 (2005) 4161–4171, https://doi.org/ 10.1002/pola.20869.
[31] N. Joseph, P. Ahmadiannamini, R. Hoogenboom, I.F.J. Vankelecom, Layer-by- layer preparation of polyelectrolyte multilayer membranes for separation, Polym. Chem. 5 (2014) 1817–1831, https://doi.org/10.1039/C3PY01262J
[32]H. Xu, H. Shi, Y. Yang, X. Liu, Synthesis and characterization of nanocomposites Fe3O4–SiO2–chitosan based on lbl technology, Glass. Phys. Chem. 42 (2016) 312–321, https://doi.org/10.1134/S1087659616030056.
[33] H. Gao, O.A. Goriacheva, N.V. Tarakina, G.B. Sukhorukov, Intracellularly biodegradable polyelectrolyte/silica composite microcapsules as carriers for small molecules, ACS Appl. Mater. Interfaces. 8 (2016) 9651–9661, https://doi. org/10.1021/acsami.6b01921.
[34] B. Tieke, F. van Ackern, L. Krasemann, A. Toutianoush, Ultrathin self-assembled polyelectrolyte multilayer membranes, Eur. Phys. J. E. 5 (2001) 29–39, https:// doi.org/10.1007/s101890170084.
[35] R. Klitzing, B. Tieke, Polyelectrolyte Membranes, in: Springer Berlin Heidelberg, 2004, pp. 177–210. doi: 10.1007/b11270.
[36] L. Krasemann, B. Tieke, Ultrathin self-assembled polyelectrolyte membranes for pervaporation, J. Membr. Sci. 150 (1998) 23–30, https://doi.org/10.1016/ S0376-7388(98)00212-9.
[37] B. Toutianoush, A., Tieke, Pervaporation separation of alcohol/water mixtures using self-assembled polyelectrolyte multilayer membranes of high charge density, Mater. Sci. Eng., C. 22 (2002) 459–463, https://doi.org/10.1016/S0928- 4931(02)00189-3.
[38] J. Bassil, H. Alem, G. Henrion, D. Roizard, Tailored adhesion behavior of polyelectrolyte thin films deposited on plasma-treated poly(dimethylsiloxane) for functionalized membranes, Appl. Surf. Sci. 369 (2016) 482–491, https://doi. org/10.1016/j.apsusc.2016.01.146.
[39] A.V. Penkova, S.F.A. Acquah, M.E. Dmitrenko, M.P. Sokolova, M.E. Mikhailova, E.S. Polyakov, et al., Improvement of pervaporation PVA membranes by the controlled incorporation of fullerenol nanoparticles, Mater. Des. 96 (2016) 416–423, https://doi.org/10.1016/j.matdes.2016.02.046.
[40] M.E. Dmitrenko, A.V. Penkova, A.B. Missyul, A.I. Kuzminova, D.A. Markelov, S.S. Ermakov, et al., Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation, Sep. Purif. Technol. 187 (2017) 285–293, https://doi.org/10.1016/j. seppur.2017.06.061.
[41] A.V. Penkova, S.F.A. Acquah, M.P. Sokolova, M.E. Dmitrenko, A.M. Toikka, Polyvinyl alcohol membranes modified by low-hydroxylated fullerenol C60 (OH)12, J. Membr. Sci. 491 (2015) 22–27, https://doi.org/10.1016/j. memsci.2015.05.011.
[42] A.V. Penkova, S.F.A. Acquah, M.E. Dmitrenko, B. Chen, K.N. Semenov, H.W. Kroto, Transport properties of cross-linked fullerenol–PVA membranes, Carbon N. Y. 76 (2014) 446–450, https://doi.org/10.1016/ j.carbon.2014.04.053.
[43] A.V. Penkova, S.F. Acquah, L.B. Piotrovskiy, D.A. Markelov, A.S. Semisalova, H.W. Kroto, Fullerene derivatives as nano-additives in polymer composites, Russ. Chem. Rev. 86 (2017) 530–566, https://doi.org/10.1070/RCR4712.
[44] A.V. Penkova, M.E. Dmitrenko, S.S. Ermakov, A.M. Toikka, D. Roizard, Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation, Environ. Sci. Pollut. Res. (2017) 1–9, https://doi.org/10.1007/s11356-017-9063-9.
[45] R.W. Baker, Membrane Technology and Applications, Wiley, 2004.
[46] E.V. Gribanova, M.I. Larionov, Application of contact angle dependence on pH for estimation of acid-base properties of oxide surfaces, Vestn. Saint-Petersbg. Univ. Ser. 4, Phy. Chem., 1401, 2014.
[47] I.Iu. Prosanov, A.A. Matvienko, Investigation of the thermal decomposition of PVA by IR and Raman spectroscopy, Phys. Solid State. 52 (2010) 2056–2059.
[48] L.A. Kazitsina, N.B. Kupletskaia, Application of UV, IR and NMR Spectroscopy in Organic Chemistry, Higher School, Moscow, 1971.
[49] D.J. Upadhyay, N.V. Bhat, Pervaporation studies of gaseous plasma treated PVA membrane, J. Membr. Sci. 239 (2004) 255–263, https://doi.org/10.1016/j. memsci.2004.03.041.